RESOURCE LIBRARY
Content and guides for more information on printing and converting processes.
free downloadable guides
OPTIMIZING YOUR MANUFACTURING CELLS
Many companies strive for lean manufacturing to increase efficiencies, reduce risk, enhance quality, reduce waste, and other desirable outcomes. This guide covers the benefits and limitations of organized manufacturing cells, typical implementation and techniques, and the difference between in-house and outsourced manufacturing.

MEMBRANE SWITCH PROCESS DESIGN GUIDE
As a trusted custom converter and OEM manufacturer, Tapecon has helped clients in a wide range of industries design, test and produce high-quality, cost-effective membrane switches. This guide will assist you in membrane switch design considerations that will help relate your product engineering to our production processes.
The Sustainability Guide
As a company with tradition rooted in innovation, Tapecon has been actively involved in a number of sustainability initiatives. In this guide, we’ll cover best practices for materials selection, printing, and converting processes, and explain how companies can focus on the triple bottom line – profit, people, and the planet.


A CASE STUDY IN OUTSOURCED MANUFACTURING
Learn more about how an active package manufacturer successfully delegated its converting and printing operations to Tapecon in just ten months realizing the following benefits:
Customer Resources
Sustainability initiative
As a company with tradition rooted in innovation, Tapecon has been actively involved in a number of sustainability initiatives. Continuous improvement has been one of Tapecon’s core values since the business was first started in 1919 and as a result, Tapecon has embraced a number of lean and sustainable practices in order to evolve.
Certifications
ISO 9001:2015
We strive to exceed our customers’ quality expectations. “Good enough” is never good enough for us. We have shown our commitment to quality by mandating that all aspects of our production and office facility conform to ISO 9001:2015 standards.
Quality Management System – ISO-9001.2015 Certificate of Registration
ISO 13485:2016
ISO 13485:2016 certification provides Tapecon with the ability to create medical devices and solutions for medical devices that consistently meet or exceed customer and regulatory requirements.
Quality Management System – ISO 13485.2016 Certificate of Registration
Customer Satisfaction Survey
We combine science and application expertise to solve the application challenges of our customers to improve their products. Our purpose delivers better product solutions that enhance people's lives. How have we helped you? Let us know by filling out this short survey.
Blog Posts
We regularly publish content to serve our fellow colleagues in the advanced manufacturing industry.
26Jan
How a Label Estimator Helps You Save Time and Money Before You Ever Request a Quote
.png)
08Jan
Traceability Made Easy: Built-In Serialization for a Clean Device History

19Dec
Driving Innovation Through Collaboration: Why 3M Solutions Seminars Matter
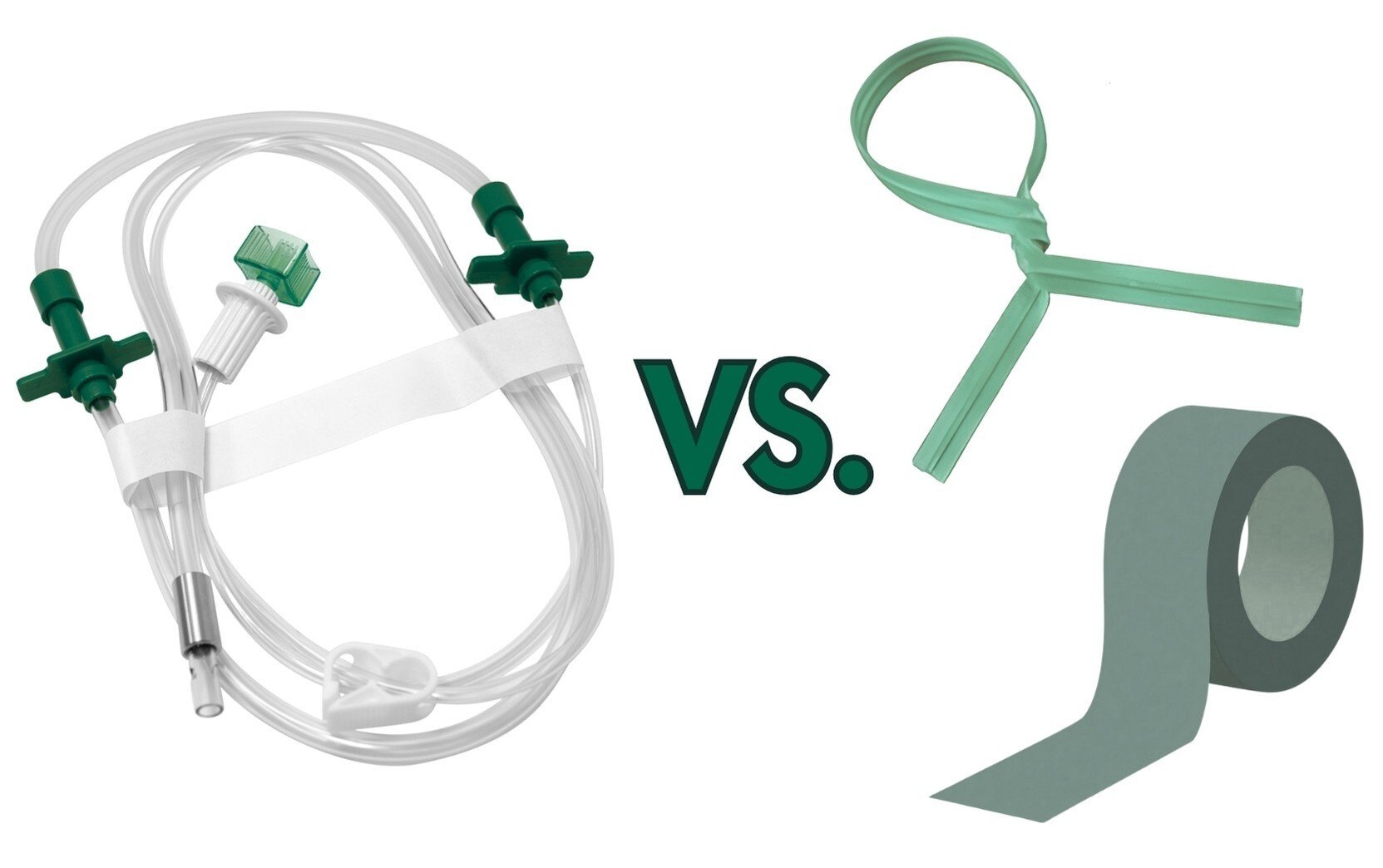
05Dec
How Ryandra™ Cohesive Tape Outperforms Adhesive Tape and Twist Ties in Medical Tubeset Packaging
.png)
20Nov
How EMI/RFI Shielding Can Lower Total Product Costs Over Its Lifecycle
.png)
07Nov
7 Real-World Ways Printed Chemical Indicators Improve Product Safety and Performance
Podcasts
On Tapecon’s Better Product Solutions Podcast, we have insightful conversations with engineers, product managers, procurement managers, and a variety of professionals in the manufacturing space to explore a simple pursuit – how we can make products better.

Podcast: Jack Anderson - 3M Activation Marketer: Industrial Tapes and Adhesives
| December 9, 2025
In this episode, Steve Davis sits down with Jack Anderson, Activation Marketer at 3M’s Industrial Adhesives and Tapes Division, to discuss the 3M..

Podcast: Michael Muchin - Business Director at Avery Dennison Medical
| September 23, 2025
In this episode, Steve Davis sits down with Michael Muchin, Business Director at Avery Dennison Medical, to explore the complexities of wearable..

Podcast: Harald Wahl Breivik, CEO at CondAlign AS
| February 11, 2025
In this episode, we chat with Harald Breivik, CEO of Condalign, a technology company based in Oslo, Norway, that focuses on developing and..

Podcast: Ken Schwartz, Head of Growth and Innovation at Covestro
| December 17, 2024
In this episode, we chat with Ken, Head of Growth and Innovation at Covestro, a leading company in the development of polycarbonate and thermoplastic..

Podcast: Peter Panneri, Business and Product Manager at Ryandra Inc.
| October 28, 2024
In this episode, we chat with Peter Panneri, Business and Product Manager of Ryandra Inc., a subsidiary of Tapecon, specializing in cohesive tape..
Podcast: Cameron Bell, Design and Application Engineer at Delta ModTech
| October 2, 2024
In this episode, we chat with Cameron Bell, Design and Application Engineer at Delta ModTech, a long-term machine collaboration partner of Tapecon...
Let's get started. Book a Meeting with Us.
To learn more about outsourced manufacturing, fill out this short form and a Tapecon representative will follow up with you as quickly as possible. As a fifth-generation family business, we pride ourselves on being trusted manufacturing partners for our clients, and we look forward to working with you.
